Abstract
Objectives: Tyrosine kinase inhibitors (TKIs) have greatly improved treatment outcomes for patients with newly diagnosed chronic myelogenous leukemia in chronic phase (CML-CP), transforming it into a chronic disease requiring long-term treatment. This emphasizes the need for treatments that combine high efficacy with a favorable safety profile, improving over currently available options.
Asciminib is a first-in-class BCR::ABL1 inhibitor specifically targeting the ABL myristoyl pocket (STAMP) which has been approved by the FDA with subsequent approvals worldwide for patients with Philadelphia chromosome-positive (Ph+) CML-CP, previously treated with ≥2 prior TKIs based on the results of the ASCEMBL (NCT03106779) trial. The ABL specificity of asciminib compared with the selectivity of second-generation (2G) TKIs can reduce off-target effects and improve tolerability while maintaining efficacy and reducing the probability of developing resistance. The ongoing ASC4FIRST (NCT04971226) study will evaluate the efficacy and safety of asciminib vs investigator-selected TKI in patients with newly diagnosed Ph+ CML-CP. Additional data on asciminib in newly diagnosed Ph+ CML-CP may help to address patient-centric objectives, in particular the ability of patients to remain on treatment without having to permanently discontinue due to adverse events (AEs).
The ASC4START study (NCT05456191) will primarily evaluate the tolerability of asciminib vs the 2G TKI nilotinib in adult patients with newly diagnosed Ph+ CML-CP, as measured by the time to study treatment discontinuation due to adverse event (TTDAE). The study will also report on further safety, efficacy and quality of life (QoL) outcomes.
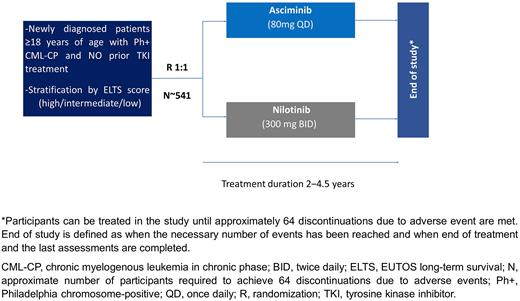
Methods: This is a phase IIIb, multi-center, open-label, randomized study in patients ≥18 years of age with newly diagnosed Ph+ CML-CP. The trial will enroll approximately 541 patients with CML-CP diagnosed within 3 months of study entry and an Eastern Cooperative Oncology Group (ECOG) performance status ≤1. Patients cannot be included if they have received previous treatment for CML with any other anticancer agents (except hydroxyurea and/or anagrelide), have confirmed central nervous system infiltration, or present impaired cardiac function or abnormalities in cardiac repolarization.
Patients will be randomized 1:1 to receive asciminib 80 mg once daily or nilotinib 300 mg twice daily, both administered orally under fasting conditions; randomization will be stratified based on European Treatment Outcome Study long-term survival (ELTS) score at diagnosis (high vs intermediate vs low) to help achieve balance between the treatment arms (Figure).
The primary endpoint is TTDAE, defined as the time from the date of the first dose of study treatment to the date of discontinuation of study treatment due to an AE. Secondary endpoints include type, frequency and severity of AEs; dose modifications due to AE; changes in laboratory values that fall outside pre-determined ranges; clinically notable ECG changes; time to discontinuation due to selected reasons (lack of efficacy/treatment failure/disease progression/suboptimal response/death); major molecular response (MMR), MR4, MR4.5, complete hematological response (CHR), and BCR::ABL1 ≤1% at and by all scheduled data collection time points; duration of and time to first MMR, MR4 and MR4.5; time to treatment failure; event-free survival, progression-free survival and overall survival. Secondary endpoints related to QoL include change from baseline in overall scores and individual scales of the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaires QLQ-C30 and QLQ-CML24.
Participants will be treated in the study until approximately 64 discontinuations due to AEs are met; treatment duration for an individual patient is expected to be 2‒4.5 years.
Current status: The study is recruiting as of October 2022.
This study was sponsored by Novartis Pharmaceuticals.
Disclosures
Hochhaus:Bristol Myers Squibb: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Incyte: Research Funding. Saussele:Incyte: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Roche: Honoraria; Pfizer: Honoraria. Mahon:Novartis: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Honoraria; Pfizer: Honoraria. Pilipovic:Novartis: Current Employment. Zhang:Novartis: Current Employment. Schuld:Novartis: Current Employment. Brümmendorf:Merck: Consultancy, Other: travel support; Pfizer: Consultancy, Honoraria, Other: travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: travel grant, Research Funding; Janssen: Consultancy, Other: travel support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal